Occurrence of metals
A native metal is any metal that is found in its metallic form in nature, either pure or as an alloy. Metals that can be found as native deposits singly and/or in alloys include antimony, arsenic, bismuth, cadmium, chromium, cobalt, indium, iron, nickel, selenium, tantalum, tellurium, tin, titanium, and zinc.
Non-metals
A non-metal is an element which ionizises by gain of electron. It gains one or more electrons when energy is given. Non-metals have 4, 5, 6, 7 valence electrons and tends to form negative ions on ionization and are electrovalent or covalent compounds. Examples include oxygen, fluorine, argon etc.
Electrovalency
In this method, one atom loses electrons and another atom gains the same amount of electrons to attain the noble gas configuration. For example, in sodium chloride, the electronic configuration of sodium is 2, 8, 1 and the electronic configuration of chlorine is 2, 8, 7. Sodium loses one electron to attain the noble gas configuration and the chlorine gains one electron to attain the argon gas configuration.
Formation of electrovalent compounds
Chemical bond formed between two atoms due to transfer of electron(s) from one atom to the other atom is called "Ionic bond" or electrovalent bond. Electrovalent compounds are formed by the transfer of electrons from one to another ion resulting in the completion of octet.
Sodium chloride
Sodium loses one electron to attain the noble gas configuration of neon. Chlorine gains this one electron to attain the noble gas configuration of argon. Sodium ions have the formula while chloride ions have the formula . Thus, an ionic bond is formed between ions and ions.
Magnesium chloride

Magnesium forms ionic bond with chlorine by donating it's two valence electrons to two Cl atoms. Magnesium loses 2 electrons and attains the noble gas configuration of neon. Each chlorine atom gains one electron to form the noble gas configuration of argon. The opposite charges of the magnesium ions and chloride ions attract each other and ionic bond is formed.
General characteristics of metals
Physical properties: 1) Malleable: material is one that can be hammered or rolled into flat sheets and other shapes.
2) Ductile: material is one that can be pulled out, or drawn, into a long wire.
3) Conductivity: It is the ability of an object to transfer heat or electricity to another object.
Chemical properties:1) Reaction with Oxygen:
Reaction with water:
+ heat
Elements in the middle of the periodic table have two electrons. It consists of heavy metals.
Elements on the right of the periodic table have 4, 5, 6, 7 electrons.
Elements on the extreme have eight electrons. They are called inert gases or noble gases
2) Ductile: material is one that can be pulled out, or drawn, into a long wire.
3) Conductivity: It is the ability of an object to transfer heat or electricity to another object.
Chemical properties:1) Reaction with Oxygen:
Reaction with water:
+ heat
Elements in the middle of the periodic table have two electrons. It consists of heavy metals.
Elements on the right of the periodic table have 4, 5, 6, 7 electrons.
Elements on the extreme have eight electrons. They are called inert gases or noble gases
Extraction of metals which are in the middle of activity series
Metals such as iron, zinc, lead, copper, etc., are in the middle of the reactivity series. These are moderately reactive metals and are usually present as sulphides or carbonates. A metal is obtained from its ore by the processes of reduction or by electrolysis. In the reduction process, it is the oxide ore that is reduced.
It is easier to reduce an oxide ore as compared to its sulphides and carbonates. If the ore is not an oxide ore, it is first converted to the oxide by the process of calcination or by roasting.
It is easier to reduce an oxide ore as compared to its sulphides and carbonates. If the ore is not an oxide ore, it is first converted to the oxide by the process of calcination or by roasting.
Extraction by carbon
Metals such as zinc, iron and copper are present in ores as their oxides. Each of these oxides is heated with carbon to obtain the metal.The metal oxide loses oxygen, and is therefore reduced. The carbon gains oxygen, and is therefore oxidised.
Using iron as an example:
iron oxide + carbon iron + carbon dioxide
Using iron as an example:
iron oxide + carbon iron + carbon dioxide
Extraction of metals which are towards the top of the reactivity series
Metals such as sodium, magnesium, calcium, aluminium high up in the reactivity series are very reactive and cannot be obtained from their compounds by heating with carbon. This is because these metals have more affinity for oxygen than carbon. These metals are obtained by electrolytic reduction.
For Na, K, Ca, Mg, Al, all these metals cannot be reduced by coke or carbon monoxide. Electrolytic method is the only way to reduce these metals
For Na, K, Ca, Mg, Al, all these metals cannot be reduced by coke or carbon monoxide. Electrolytic method is the only way to reduce these metals
Extraction by electrolysis
Metals which are highly reactive in nature in such as aluminium are extracted by the electrolysis method. Less-reactive metal such as iron may be extracted by reduction with carbon or carbon monoxide.
Extraction by electrolysis:
Reaction takes place during the electrolysis are:
Cathode:
Anode:
Extraction by electrolysis:
Reaction takes place during the electrolysis are:
Cathode:
Anode:
Methods involved in extraction of lithium
The mineral forms of lithium are heated to a high enough temperature (1200 K - 1300 K) in order to crumble them and thus allow for subsequent reactions to more easily take place. The use of sulfuric acid and sodium carbonate to allow the iron and aluminum to precipitate from the ore, from there, more sodium carbonate is applied to the remaining material allow the lithium to precipitate out, forming lithium carbonate. This is treated with hydrochloric acid to form lithium chloride.
Difficulties during extraction of alkali metals
The production of pure alkali metals is difficult due to their extreme reactivity with commonly used substances, such as water. The alkali metals are so reactive that they can not be displaced by other elements and must be isolated through high-energy methods such as electrolysis.
Methods of extraction of sodium

Down's process is the process of extracting sodium by the electrolysis of molten sodium chloride. The Downs cell has a central graphite anode surrounded by a cylindrical steel cathode. This process is based on the electrolysis of fused NaCl. Down's cell consists of a rectangular container of steel. Inside of the tank is lined with firebricks.
Chemical properties of Metals and Non-metals
| Metals | Non-Metals |
| Metals are good reducing agents | Non-metals are good oxidising agents |
| Active metals react with dilute acids to yield | Non-metals do not react with dilute acids |
| They form basic oxides and are electrovalent | They form acidic oxides and they are covalent |
| Metallic atoms donate electrons to form electrovalent chlorides | They form covalent chlorides. The chlorides of non-metals are insoluble in water |
Refining
Refining consists of purifying an impure material, in this case a metal. It is to be distinguished from other processes such as smelting and calcining in that those two involve a chemical change to the raw material, whereas in refining, the final material is usually identical chemically to the original one, only it is purer.
Electrolytic refining
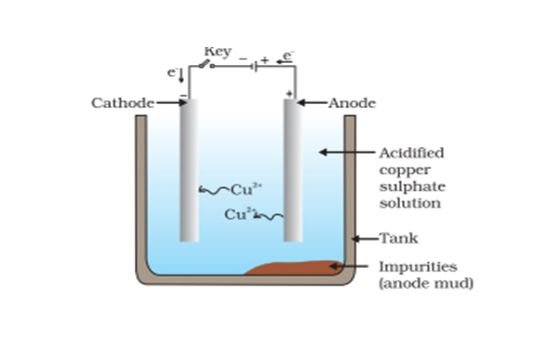
Electrolytic refining: It is the process of refining of metal in which impure metal is made the anode and a thin sheet of pure metal is made the cathode of an electrolytic cell containing an aqueous solution of the metal salt. When an electric current of a suitable voltage is passed, impure metal at the anode gets dissolved to deposit the pure metal at the cathode.
Metal ions from the anode enter the electrolyte as follows:
The impurities are left behind as anode mud near the anode. The anode finally disintegrates while the cathode gains in weight due to the collection of pure metal.
Metal ions from the anode enter the electrolyte as follows:
The impurities are left behind as anode mud near the anode. The anode finally disintegrates while the cathode gains in weight due to the collection of pure metal.







No comments:
Post a Comment