Describe carbon
1. Carbon is an element with atomic number 6 with symbol C.
2. Carbon shows hybridization hence its valence is 4 and it is called as tetravalent.
3. Carbon is most commonly obtained from coal deposits, although it usually must be processed into a form suitable for commercial use.
4. Three naturally occurring allotropes of carbon are known to exist: amorphous, graphite and diamond.
5. Carbon present in saturated form eg. Alkane where it contain single bond.
6. Carbon in unsaturation shows double and triple bond eg. Alkene and Alkyne.
2. Carbon shows hybridization hence its valence is 4 and it is called as tetravalent.
3. Carbon is most commonly obtained from coal deposits, although it usually must be processed into a form suitable for commercial use.
4. Three naturally occurring allotropes of carbon are known to exist: amorphous, graphite and diamond.
5. Carbon present in saturated form eg. Alkane where it contain single bond.
6. Carbon in unsaturation shows double and triple bond eg. Alkene and Alkyne.
Allotropes of carbon
Allotropy is the property of an element to exist in more than one physical forms having similar chemical properties but different physical properties. Carbon exists both in crystalline and amorphous allotropic forms. Crystalline allotropes of carbon: diamond, graphite and fullerene. Amorphous allotropes of carbon: coal, coke, charcoal, lampblack, gas and coke.
Properties of ethanol
- Ethyl alcohol is a colorless liquid with boiling point 351 K.
- It has a characteristic smell and burning taste.
- It is miscible in water.
- It is good solvent for fats , oils, paints etc.
- Ethyl alcohol is typical monohydric alcohol gives all general monohydric alcohol reactions.
Condensed structural formula of alkyl and functional group
1. Condensed structural formula represent structure of organic compound written in condensed form or short form in which same repeating groups are condensed in one group. Example : can be written as following in condensed form .
Nomenclature for compounds containing one fuctional group
1.The longest continuous chain containing the carbon atoms involved in the multiple bond, functional groups or substituents is selected as a parent chain.
2. While writing the name of the alkene or alkyne, the suffix -ane of the corresponding alkane is replaced by -eneor -yne respectively, same for functional group.
3. If the multiple bond occurs twice in the parent chain, the alkene and alkyne containing the double or triple bond gets the lowest number.
All the rules for naming the side chains or substituents are similar to alkanes.
2. While writing the name of the alkene or alkyne, the suffix -ane of the corresponding alkane is replaced by -eneor -yne respectively, same for functional group.
3. If the multiple bond occurs twice in the parent chain, the alkene and alkyne containing the double or triple bond gets the lowest number.
All the rules for naming the side chains or substituents are similar to alkanes.
Substitution reaction of carbon compounds
A reaction in which an atom or group of atoms replaces another atom or group of atoms is called substitution reaction. Alkanes undergo substitution reactions. Example: Chlorination of methane in presence of sunlight gives a mixture of products like methyl chloride, methylene chloride, chloroform and carbon tetrachloride.
Combustion of carbon compounds
All carbon compounds react with oxygen to produce heat and light along with carbon dioxide and water. This reaction of carbon with oxygen is called combustion. Aliphatic compounds on combustion produce a non-sooty flame. Aromatic compounds on combustion produce sooty flame.
Oxidation of carbon compounds
1. Oxidation is a reaction in which carbon compounds get oxidized by oxidizing agents into compound with more number of oxygen atom
2. For example : Alcohols undergo oxidation in presence of oxidizing agents like alkaline potassium permanganate or acidified potassium dichromate to form carboxylic acids
3. Ethyl alcohol on oxidation with alkaline potassium permanganate or acidified potassium dichromate gives acetic acid
2. For example : Alcohols undergo oxidation in presence of oxidizing agents like alkaline potassium permanganate or acidified potassium dichromate to form carboxylic acids
3. Ethyl alcohol on oxidation with alkaline potassium permanganate or acidified potassium dichromate gives acetic acid
Addition reaction of carbon compounds
A chemical reaction is said to be an addition reaction if two substances combine and form a third substance. In general, unsaturated hydrocarbons like alkenes and alkynes prefers to undergo addition reactions. In addition reactions, molecules add across double bond or triple bond. Hydrogenation reaction involves the addition of hydrogen to unsaturated hydrocarbons in presence of catalyst like nickel or platinum to form saturated hydrocarbons.
Soap as a salt
Soap consists of sodium or potassium salts of fatty acids like stearic, palmitic and oleic acids obtained by reacting fatty acid with a strong base such as Sodium Hydroxide or Potassium Hydroxide.
The sodium soaps are called hard soaps and the potassium soaps are known as soft soaps. Soaps are obtained from oils and fats.
The sodium soaps are called hard soaps and the potassium soaps are known as soft soaps. Soaps are obtained from oils and fats.
Cleansing action of soaps
Most of the dirt is oily in nature and oil does not dissolve in water. The molecule of soap constitutes sodium or potassium salts of long chain carboxylic acids. In the case of soaps the carbon chain dissolves in oil and the ionic end dissolves in water. Thus the soap molecules form structures called micelles. In micelles one end is towards the oil droplet and the other end which is the ionic faces outside. Therefore, it forms emulsion in water and helps in dissolving the dirt r when we wash our clothes. The soap is a kind of molecule in which both the ends have different properties. The first one is the hydrophilic end which dissolves water and is attracted towards it whereas the second one is the hydrophobic end that is dissolved in hydrocarbons and is water repulsive in nature. If on the surface of water, soap is present then the hydrophobic tail which is not soluble in water will align along the water surface.In water the soap molecule is uniquely oriented which helps to keep the hydrocarbon part outside the water. When the clusters of molecules are formed then hydrophobic tail comes at the interior of the cluster and the ionic end comes at the surface of the cluster and this formation is called micelle. When the soap is in the form of micelles then it has the ability to clean the oily dirt which gets accumulated at the centre. These micelles remain as a colloid in the solution. Therefore the dirt from the cloth is easily washed away. The soap solution appears cloudy as it forms a colloidal solution which scatters light.
Cleansing action of soap
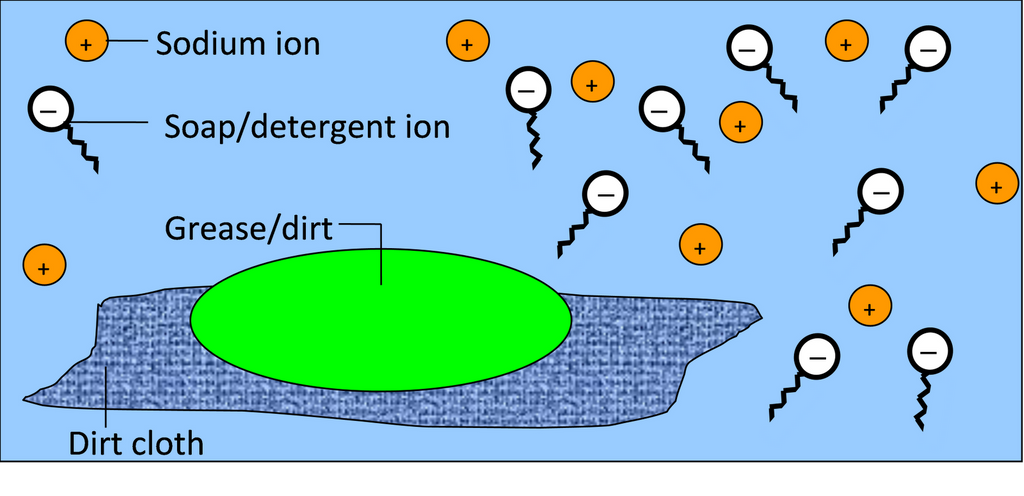
As we know that the micelle consist of a hydrophobic hydrocarbon and the soap moleculs form micelle around the oil droplets in such a way that hydrophobic part of the stearate ions is in the oil droplets and hydrophilic part projects out of the grease droplet. So the polar group can interact with water and the oil droplets surrounded by stearate ions is now pulled in water and removed from the dirty surface. Thus, soap helps in emulsification and washing away of oils and fats.
Cleansing action of detergents
Detergents are a type of surface active agent or surfactant that consists of a hydrophobic "tail" and a hydrophilic "head". In aqueous solution the surfactant molecules tend to form "micelle" structures in order to keep their tails together and away from the solution phase. It is possible for the oily molecules in most dirt to enter the centre of these micelles and therefore be effectively dispersed in the water and washed away. This process proceeds much more rapidly with some mechanical action which is why scrubbing, mixing etc. is usually required.
Advantages of detergents over soap
- Synthetic detergents can be used in hard water without any wastage while soap gets wasted in water.
- Synthetic detergents can be used in acidic medium while soaps get precipitated in acidic medium.
- Synthetic detergents decrease the surface tension of water to greater extent and hence have stronger cleansing action than soaps.




No comments:
Post a Comment